She subsequently wrote her doctoral thesis, with Gigault as supervisor, on the subject of how and where micro- and nanoplastics accumulate in porous materials. As she neared the end of this project, she was astounded to discover that large quantities of microplastics had also accumulated in the Arctic sea ice. Studies had confirmed this shortly before. Ice is a porous substance; it has areas of higher and lower density, as well as cavities and microscopic saltwater flows between the ice crystals. As a result, there is a constant exchange between the seawater and the ice – and Pradel’s interest in this phenomenon began to grow. “Micro- and nanoparticles can get lodged between the ice crystals. This is highly problematic, as these are precisely the places where microalgae thrive best,” she explains. Other researchers have shown that these algae absorb toxic plastic additives, thereby granting them entry to the Arctic food chain.
A study from 2018 showed that it is the smallest microplastic particles that are the most common in sea ice. By definition, microplastics are smaller than 5 centimeters, and nanoplastics smaller than 1 micrometre. Researchers can’t quantify plastic particles smaller than 10 micrometres, which is the analytical limit. “This suggests that we can neither see nor accurately measure the lion’s share of the plastic present in the sea ice,” Pradel says.
Postdoc work for better analytics
While working on her doctoral thesis, Pradel developed a method for growing sea ice in the lab. Since April 2022, she has been cultivating these ice cores as part of a postdoctoral fellowship at the Department of Environmental Sciences at ETH Zurich. The first step of her method is to cool seawater in a glass column with a temperature gradient ranging from 1°C (lower end) to −5°C (upper end). After 19 hours, an ice core some 10 centimetres thick forms at the upper end. If micro- and nanoplastic particles are added to the seawater at the beginning, Pradel can track how the particles get from the water into the ice, where they remain.
Today, Pradel conducts research within the group led by Professor Denise Mitrano, whom she met at a conference. Mitrano’s group researches anthropogenic particles and their toxicity and impact on the environment. Among other things, she has developed analytical methods that allow them to measure micro- and nanoplastics much more precisely – the ideal complement to Pradel’s research. A central problem in the quantification of micro- and nanoplastics is to distinguish the carbon in natural materials, such as algae, from that in plastic. The researchers can circumvent this problem by adding inorganic tracers to the plastic particles. These tracers are, fittingly, trace elements, which serve as proxies for the plastics and make it possible to effectively measure the plastic particles in the ice using standard environmental analysis methods, including inductively coupled plasma mass spectrometry.
First Arctic expedition
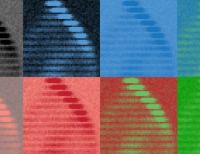
For her analyses, Pradel also collaborates with researchers from the Swiss Federal Institute for Forest, Snow and Landscape Research (WSL). She uses tomography at the WSL laboratory in Davos to analyse her ice cores at −15°C, and the resulting images provide data on the porosity and structure of the ice. “This gives us important information about where micro- and nanoplastic particles accumulate,” Pradel says. Current experiments show that nanoplastic particles are transported through the ice in a similar way to salts dissolved in seawater. How microplastics accumulate in sea ice, by contrast, depends more on the particles’density.